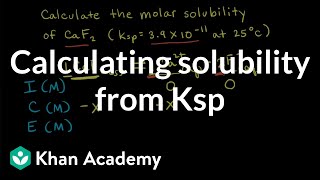
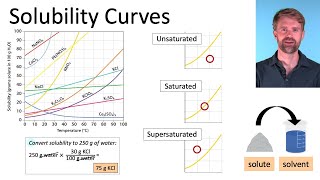
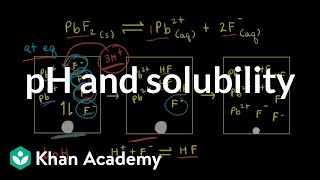
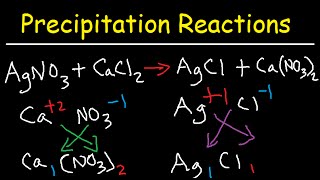
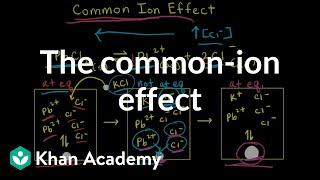
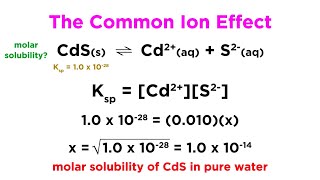
How to Calculate Ksp Silver Oxalate from Solubility? |

|
|
ksp solubility product constant, Calculating Ksp From Molar Solubility - Solubility Equilibrium Problems - Chemistry, Ksp Chemistry Problems - Calculating Molar Solubility, Common Ion Effect, pH, ICE Tables, What is Ksp? (Solubility Product Constant), How To Calculate Molar Solubility From Ksp - Solubility Product Constant, Ice Tables, Chemistry
Chemical Equilibrium Playlist: https://www.youtube.com/watch?v=ouz6PK6Mv-0&list=PLDwv-O7TJyNjZ-RNkzyAwxKc8a_R9ZqKM Chemistry Playlist:https://www.youtube.com/watch?v=qUllTDm99kY&list=PLDwv-O7TJyNgT7IvoJdsQ40WYDYLTDHo7 Solubility Playlist: https://www.youtube.com/watch?v=-WgdROvM620&list=PLDwv-O7TJyNiDATf6f0-gXzSZG1ZJM4L2 Precipitation and Solubility Playlist: https://www.youtube.com/watch?v=3gP1VpcK4OE&list=PLDwv-O7TJyNgFtCwX8SspCiOkHeAjmuu3 |