How to Find the Number of Atoms in Al2O3 (Aluminum oxide) |

|
|
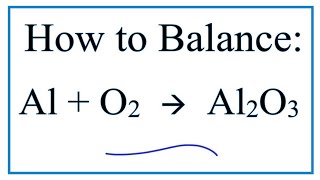
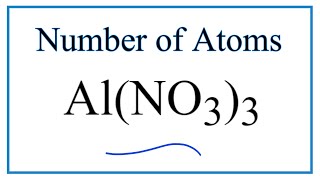
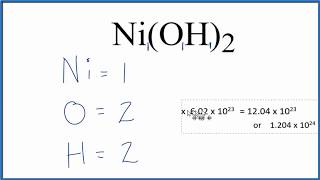
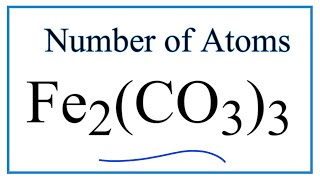
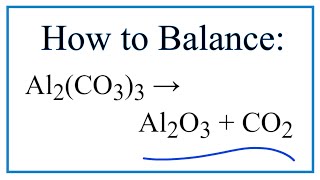
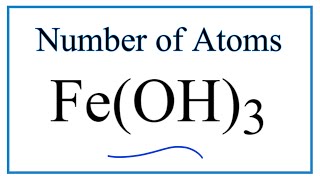
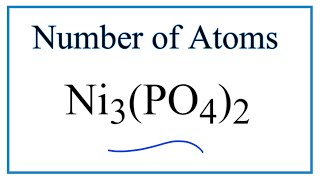
To find the total number of atoms in Al2O3 (Aluminum oxide) we’ll add up the number of each type of atom. The small number after the element symbol is called the subscript and this tells us how many of that atom are in the compound. For example, H2 means there are two Hydrogen atoms.
When there is no number after an element, we assume there to be one atom of that element. For example, in H2O we have only one Oxygen atom since there isn’t a number after the O. If there is a number in front of the compound (it’s called a “coefficient”), like 2H2O that means we have two separate molecules. We therefore we multiple the subscript for each element by the number in front. For 2H2O we have four H atoms (the coefficient 2 times the subscript 2) and two O atoms. For compounds with parenthesis, like Ca(NO3)2, we multiple the atoms inside the parentheses by the subscript. In this case we would have two N atoms and six O atoms. There is only one Ca atom. Finally, if you are asked to find the number of atoms in one mole, for example, the number of H atoms in one mole of H2O, you multiply the number of atoms by Avogadro’s number. In this case we have two H atoms so we multiply 2 x 6.02 x 10^23. Finding the number of atoms in Aluminum oxide |