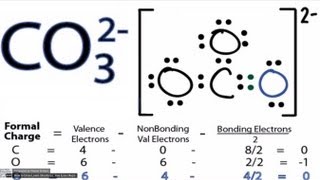
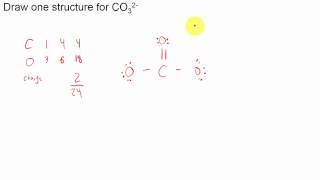
Resonance Structures for CO3 2- (Carbonate ion) |

|
|
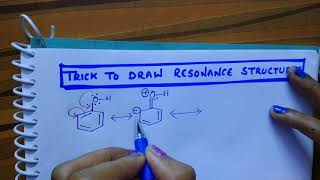
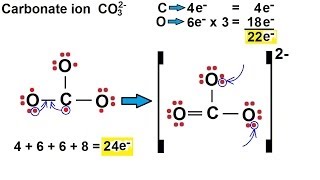
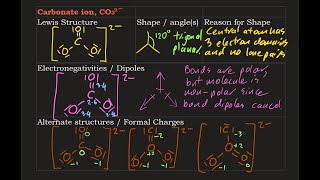
There are equivalent three resonance structures CO32-, the nitrite ion. We start with a valid Lewis structure and then follow these general rules.
- Resonance forms must be valid Lewis structures. - Maintain the same number of valence electrons. - Atoms don’t move. Only electrons move. - Only move electrons in lone pairs or pi bonds (found in double and triple bonds). - Maintain the same number of lone pairs. Remember: - Resonance structures are necessary to show how electrons are distributed in chemical bonds in a molecule. - Understand the molecule isn't flipping back and forth between structures! It's an average of the resonance structures. - The double arrow symbol drawn between resonance structures does not mean equilibrium or any sort of change. It only shows that there is more than one way to draw the structure. It's not a very good choice of symbols, really. Resonance structures are also called resonance forms, resonance contributors, and sometimes resonance canonicals. More chemistry help at http://www.Breslyn.org. |














!["examples of Resonance" [ Carbonate ion & sulphate ion]](https://ytimg.googleusercontent.com/vi/U8R45tJEjlE/mqdefault.jpg)