Is HClO3 (Chloric acid) Ionic or Covalent/Molecular? |

|
|
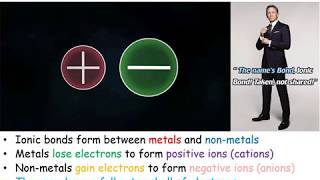
Is HClO3 (Chloric acid) Ionic or Covalent/Molecular? To tell if HClO3 is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cl and O are non-metals and H is also a non-metal. When we have all non-metals the compound is usually considered covalent.
Because we have a non-metal and non-metal in HClO3 there will be a difference in electronegativity between Cl, O, and H is less than 2.0. This difference results in an electron(s) being shared between the non-metals. The sharing means that the bond will be considered covalent/molecular. While Chloric acid is considered covalent, it is often considered a strong acid and will dissociate into H+ and ClO3- ions in water. --- Helpful Resources Metals, Non-Metals on the P- Table: https://youtu.be/OoooStZQHdA Ionic, Covalent, & Polar Covalent: https://youtu.be/OHFGXfWB_r4 Electronegativity for each element: https://en.wikipedia.org/wiki/Electronegativity --- Because we have a combination of a non-metal and non-metal HClO3 (Chloric acid) is considered an covalent/molecular compound. In general, covalent compounds compounds: - have low melting points and boiling points. - have low enthalpies of fusion and vaporization. - do not conduct electricity when dissolved in water. For more chemistry help, see http://www.Breslyn.org. |