How to Calculate the Formal Charges for CO3 2- (Carbonate ion) |

|
|
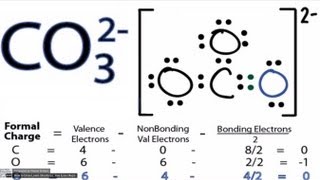
In order to calculate the formal charges for CO3 2- we'll use the equation:
Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding electrons / 2] The number of valence electrons for the atom of interest is found on the Periodic Table. We find the number of bonding and nonbonding electrons from the Lewis Structure for CO3 2- . How to draw the CO3 2- Lewis Structure: https://youtu.be/vheqX9W8bf4 Nonbonding valence electrons are those around the atom of interest that are not involved in chemical bonds (they aren't being shared with another atom). Bonding valence electrons are the ones shared between atoms. We'll divide this number by two. For more help with chemical bonding and formal charge visit http://www.Breslyn.org Some things to note about CO3 2- Formal Charges: - Formal charge is different from the oxidation number! - If you can exceed the octet rule for the central atom it's a good idea to check the formal charges. - If we have isomers or resonance -- formal charges will help us determine most stable structure. - The closer the formal charges are to zero the more likely we have the most favorable Lewis structure for the molecule. - We write the formal charges in (). E.g. (-1) Helpful Videos: • Formal Charges: https://youtu.be/vOFAPlq4y_k • Finding Valence Electrons (element): https://youtu.be/x1gdfkvkPTk • Exceptions to the Octet Rule: https://youtu.be/Dkj-SMBLQzM • How to Draw Lewis Structures: https://youtu.be/1ZlnzyHahvo • Practice Lewis Playlist: https://www.youtube.com/playlist?list=PLZR1BGWBaZ1wEg1Z22ksfNo34UUbaFORo Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). |