How to Balance: Na + Cl2 = NaCl |

|
|
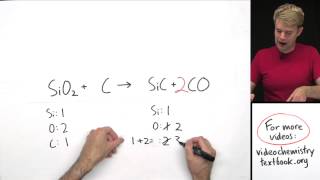
This is a classic chemical equation used to demonstrate balancing. Visit https://www.Breslyn.org for video guides on balancing equations and more!
In order to balance Na + Cl2 = NaCl you'll need to make sure the number of Na and Cl atoms are the same on both sides of the chemical equation. You do that by changing the coefficients (the numbers in front of atoms or compounds). First, be sure to count all of Na and Cl atoms on each side of the chemical equation. Once you know how many of each type of atom you have you can only change the coefficients (the numbers in front of atoms or compounds) in order to balance the equation. Second, when you balance the equation it is easier to change the coefficient in front of NaCl first in order to get the Cl atoms on each side to match. --- Drawing/writing done in Adobe Illustrator 6.0. Screen capture done with Camtasia Studio 4.0. Done on a Microsoft Surface Pro 3. |