How to Write the Net Ionic Equation for ZnO + HCl = ZnCl2 + H2O |

|
|
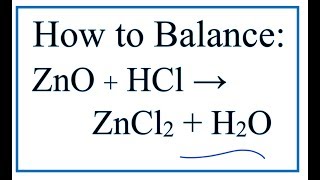
There are three main steps for writing the net ionic equation for ZnO + HCl = ZnCl2 + H2O (Zinc oxide + Hydrochloric acid). First, we balance the molecular equation.
Second, we write the states and break the soluble ionic compounds into their ions (these are the strong electrolytes with an (aq) after them). Finally, we cross out any spectator ions. These are the ions that appear on both sides of the ionic equation. If you are unsure if a compound is soluble when writing net ionic equations you should consult a solubility table for the compound. _________________ Important Skills Finding Ionic Charge for Elements: https://youtu.be/M22YQ1hHhEY Memorizing Polyatomic Ions: https://youtu.be/vepxhM_bZqk Determining Solubility: https://www.youtube.com/watch?v=5vZE9K9VaJI More Practice Introduction to Net Ionic Equations: https://youtu.be/PXRH_IrN11Y Net Ionic Equations Practice: https://youtu.be/hDsaJ2xI59w _________________ General Steps: 1. Write the balanced molecular equation. 2. Write the state (s, l, g, aq) for each substance. 3. Split soluble compounds into ions (the complete ionic equation). 4. Cross out the spectator ions on both sides of complete ionic equation. 5. Write the remaining substances as the net ionic equation. Writing and balancing net ionic equations is an important skill in chemistry and is essential for understanding solubility, electrochemistry, and focusing on the substances and ions involved in the chemical reaction and ignoring those that don’t (the spectator ions). More chemistry help at http://www.Breslyn.org |